Hemodynamic observations of Tumo yoga Practitioners in a Himalayan Environment
The Journal of Alternative and Complementary Medicine // 2014, Vol.20, No4, p.295-299.
doi:10.1089/acm.2013.0159.
Rinad S. Minvaleev, PhD, Saint-Petersburg State University, Saint-Petersburg, Russia.
Alfred R. Bogdanov ,MD, PhD, Institute of Nutrition, Moscow, Russia.
Rinat R.Bogdanov MD, PhD, Regional Clinical Institute, Moscow, Russia.
David P. Bahner MD, RDMS, Columbus, Ohio, USA.
Paul E. Marik, MD, FCCP, FCCM, Norfolk, VA, USA.
Background: Few attempts have been made to evaluate the physiology of traditional Eastern health practices. The goal of this study was to evaluate the hemodynamic effects of the mysterious Buddhist practice of Tumo. Tumo is a meditative practice which produces inner heat through the alleged cultivation of body energy-channels.
Methods: This study was performed by members of an international expedition to the Himalayan Mountains in the Republic of India. The study was performed in an unpopulated outdoor mountainous area at an altitude of 16 400 ft with ambient temperatures between -10 and -15oC. Two cohorts of subjects were studies; healthy non-yogi volunteers and tumo practitioners. All the subjects were stripped down to their under-clothes and exposed to the sub-zero atmospheric temperatures for 5 minutes. The volunteers were then passively rewarmed while the tumo practitioners performed tumo for up to 10 minutes. Blood pressure, heart rate and stroke volume index (SVI) and cardiac index (CI) were measured non-invasively using a NICOM™ hemodynamic monitor, while carotid blood flow and biventricular performance were determined echocardiographically at each stage of the experiment. The total peripheral resistance index (TPRI), left ventricular ejection fraction (LVEF) and tricuspid annular plane systolic excursion (TAPSE) were determined using standard formula.
Results : Fourteen subjects completed the study, six volunteers and 8 tumo practitioners. There was one female subject in each group. With cold exposure, the SVI and carotid blood flow fell while the TPRI increased significantly in both groups. In the volunteer group these changes retuned to baseline with rewarming. Following tumo the cardiac index (4.8±0.6 vs 4.0±0.5 l/m2; p2, p
Conclusion: Tumo was associated with a hyperdyanmic vasodilated state with increased biventricular performance. We postulate that tumo results in a massive increase in sympathetic activity with activation of brown adipose tissue and marked heat production. The increased heat production may explain the paradoxical vasodilatation in tumo practitioners exposed to sub-zero temperatures.
Key words: Tumo; yoga; environmental exposure; hemodynamics; cerebral blood flow; cardiac output.
Introduction
For centuries, the West has been fascinated with the health practices of the East. When viewed by Westerners and separated from cultural traditions these practices are met with either exuberant enthusiasm or prompt dismissal. As a result, few attempts have been made to seriously evaluate the physiological effects of these traditional Eastern techniques. For the zealots such proof is unnecessary and for the nihilists no proof is possible. To remedy this situation our team decided to evaluate the hemodynamic effects of the somewhat mysterious Buddhist practice of Tumo (or Tum-mo). Tumo is the Tibetan word for inner fire. Tumo is a meditative practice which produces inner heat through the alleged cultivation of body energy-channels.(1,2) Tumo is practiced for the most part by Buddhist monks, but tumo techniques are undoubtedly rooted in the yogic practice of Agni-Sara. Tumo adepts, also known as ‘repas’ are known for their remarkable ability to tolerate cold exposure without ill effects of hypothermia or even visible discomfort. (1,2) Once qualified tumo yogis can spend hours nearly naked on the Himalayan glaciers melting ice or snow or comfortably meditating in the waterfalls by generating internal body heat. Most tumo yogis are men, but women can become repas. Repas are advised not to perform the tumo practice close to buildings, concentrations of people or other influences collectively known as “foul air” that are thought to be destructive to meditation. The description of psychic, meditative and religious aspects of Tumo is beyond the boundaries of this study and will not be discussed. We will however describe the technical aspects of the practice in necessary detail.
We have observed that tumo appears to consist of three steps or stages, though some techniques differ between practitioners; i) While sitting in a cross-legged (or Lotus) position the practitioner exhales as deeply as possible attempting to rid the lungs of as much air as he (she) can (see Figure 1). ii) Without inhaling, the anterior abdominal wall muscles are contracted and relaxed vigorously in an in-and-out or circular motion. Some, but not all practitioners will also shake the body or bounce slightly. On an average the breath is held from 1.5 to 2.5 minutes while the abdominal exercises are performed. iii) With the spine extended and head held somewhat back, deep inhalation is performed at which time it said the actual heat or sensation of warmth will occur (see video in supplementary material). These steps are repeated as necessary but on an average every 3-5 minutes to keep heat generation constant.
Study design
This study was conducted in October 2012 by members of an international expedition to the Republicof India, Himachal Pradesh province in the HimalayanMountains. A seasoned expedition organizer (RSM) gathered tumo yoga experts, scientists and physicians (certified in echocardiography). The study was performed at the Rohtang and KunzumPasses(average altitude of 5 000 m / 16 400 ft), sites where tumo has traditionally been practiced. The subjects were studied outdoors at an isolated location at subzero temperatures (-10 to -15oC with the wind chill factor). This study was approved by the institutional review board of Saint Petersburg University (irbspsu@yandex.ru). Written informed consent was translated into all relevant languages and cross translated to assure accuracy. It was signed by all participants after all questions were answered by the scientific team.
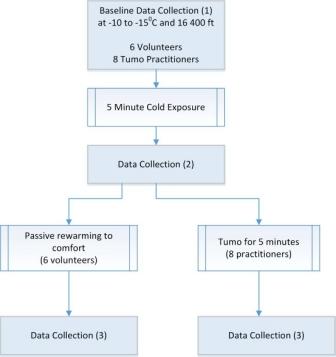
We studied two cohorts, namely i) six healthy non-yogi volunteers (V) and ii) eight tumo practitioners (T). Demographic (age/sex) and anthropometric data (height, weight, body mass index, body surface area) were recorded on each subject. With subjects sitting in the lotus position baseline hemodynamic data (detailed below) was obtained in all subjects (measurement No.1). The subjects then stripped down to their under-clothes being exposed to the sub-zero atmospheric temperatures. The second set of hemodynamic data was obtained on all subjects after 5 minutes of cold exposure (measuremen No. 2). The volunteers were then passively rewarmed for 10 minutes and the third hemodynamic profile was then obtained (measurement No. 3). The tumo practitioners re-warmed themselves by performing tumo with the third hemodynamic profile being obtained after about 10 minutes of tumo (measurement No. 3). The subjects were sequentially studied over a two day period with each experiment lasting approximately 20-25 minutes. An outline of the research process is provided in Figure 2.
Hemodynamic measurements
Two portable ultrasound units (SonoSite Edge, SonoSite, Bothell, WA) with a phased and linear array transducer and a bioreactance cardiac output (CO) monitor (NICOM, Cheetah Medical (Vancouver, WA), obtained through an in kind grant were used to obtain the hemodynamic profile. Standard echocardiographic views (parasternal long axis, parasternal short axis, substernal and 4 chamber) were obtained on all subjects at each time point. Color flow and Doppler analysis, as well as motion mode was used to obtain hemodynamic measurements at the mitral, aortic, tricuspid and pulmonic valves. Images were saved as video clips and still images for later analysis. Wave form analysis was performed to obtain all metrics used in calculating hemodynamic function and flow. In the tumo practitioners stroke volume index (SVI), left ventricular ejection fraction (LVEF) and tricuspid annular plane systolic excursion (TAPSE) were determined echocardiographically at baseline (measurement No 1) and post-tumo (measurement No 3). The biplane Simpson’s formula was used for calculation of SVI and LVEF. (3) TAPSE, a measure of right ventricular function, was measured with M-mode echocardiography.(4,5) Common carotid arterial blood flow was measured concurrently with the echocardiographic examination by a technique that we have previously described.(6) Briefly, a linear array transducer (10 MHz) was used to image the common carotid artery in long axis. Utilizing color flow, a Doppler gate was placed in the center of the vessel. The velocity time integral (VTI) was determined automatically through digitalized Doppler spectral envelopes. Vascular software allowed calculation of carotid blood flow (in ml/min) from the VTI and vessel diameter (measured from intima to intima).
NICOM® bioreactance technology provides a validated, real-time, non-invasive and simple method to dynamically measure stroke volume (SV) and CO.(7-9) Bioreactance SV measurement is based on an analysis of the relative phase shifts of an oscillating current which is applied across the thoracic cavity.(7) The system is portable (with a battery pack) and is truly non-invasive. The system consists of a high-frequency (75 kHz) sine wave generator and four dual electrode “stickers” that are used to establish electrical contact with the body. Two stickers are placed on the right side of the body, and two stickers are placed on the left side of the body (below the clavicle and on the lower lateral chest on each side). The stickers on a given side of the body are paired, so that the currents are passed between the outer electrodes of the pair and voltages are recorded from between the inner electrodes. The system’s signal processing unit determines the relative phase shift (ΔΦ) between the input and output signals. The peak rate of change of Φ (dΦ /dtmax) is proportional to peak aortic flow during each beat allowing calculation of the stroke volume.(10) The device records the heart rate and has an automated non-invasive blood pressure monitor, therefore allowing calculation of the following parameters: SV, stroke volume index (SVI), CO, cardiac index (CI), mean arterial pressure (MAP), total peripheral resistance (TPR) and total peripheral resistance index (TPRI). These parameters are updated every minute and stored in the device for later review/export.
Data analysis
The subject’s data were de-identified , abstracted, and recorded in an electronic spreadsheet (Excel 2010, Microsoft, Redmond, WA). Summary statistics were used to describe the clinical data, grouped by volunteers and tumo practitioners. Paired Student’s tests were performed to compare the hemodynamic variables at baseline (measurement No.1) with measurements No. 2 and3 ineach group. We used the Shapiro-Wilk and the Kolmogorov-Smirnov methods to test the data for normality. The correlation coefficient between the SVI measured by NICOM was compared with that measured echocardiographically. NCSS 8 (Kaysville,Utah) was used to perform the statistical analysis. Unless otherwise stated, all data are expressed as mean ± SD, with statistical significance declared for probability values of 0.05 or less.
Results:
Fourteen subjects completed the study, six volunteers and 8 tumo practitioners. There was one female subject in each group. The mean age of the volunteers was 38±12 years and that of the tumo group was 38±8 years. The body mass index (BMI) of the volunteers and tumo practitioners were 22±3.4 and 24±3.2 kg/m2respectively. All subjects were able to successfully complete all three stages of the experiment with none of the subjects shivering during any stage of the study. The subjects’ hemodynamic data at each time point is listed in Table 1. Both the volunteers and tumo practitioners demonstrated a significant fall in SVI with an increase in TPRI after exposure to cold. These changes were largely reversed in the volunteers with rewarming. In the tumo practitioners the CI, LVEF and TAPSE increasedsignificantly following the tumo maneuver while the TPRI fell. The correlation coefficient between the SVI measured by NICOM with that measured echocardiographically was 0.82 (p<0.001).
Table 1. Hemodynamic data at baseline, after 5 minutes of cold exposure (-10 to -15oc) and following rewarming or Tumo
|
Parameter |
Volunteer |
Volunteer |
Volunteer |
Tumo-Baseline (1) |
Tumo- |
Post Tumo (3) |
|
SVI (ml/m2)$ |
54 ± 10 |
48 ± 8* |
53± 11 |
58 ± 4 |
49 ± 1* |
62 ± 9 |
|
CI (l/m2) |
4.0 ± 0.5 |
3.6 ± 0.6 |
4.0 ± 0.8 |
4.0 ± 0.5 |
3.7 ± 0.5 |
4.8 ± 0.6* |
|
HR (beats/min) |
74 ± 8 |
75 ± 6 |
75 ± 4 |
71 ± 11 |
78 ± 13 |
79 ± 11* |
|
MAP (mmHg) |
97 ± 14 |
112 ± 21 |
102 ± 17 |
110 ± 15 |
116 ± 18 |
106 ± 12 |
|
TPRI (dyne-s/cm5.m2) |
1936 ± 380 |
2594 ± 550* |
2215 ± 467 |
2173 ± 281 |
2633 ± 401* |
1786 ± 189* |
|
Carotid Flow (ml/min/m2) |
384 ± 116 |
285 ± 79* |
387 ± 83 |
325 ± 100 |
269 ± 75* |
445 ± 127* |
|
SVI (ml/m2)# |
- |
- |
- |
55 ± 4 |
- |
59 ± 5 |
|
LVEF (%) |
- |
- |
- |
64 ± 7 |
- |
68 ± 5* |
|
TAPSE (cm) |
- |
- |
- |
2.4 ± 0.5 |
- |
2.9 ± 0.4* |
SVI=stroke volume index; CI=cardiac index. HR=heart rate; MAP=mean arterial pressure,; TPRI= total peripheral resistance index; LVEF =left ventricular ejection fraction; TAPSE= Tricuspid annular plane systolic excursion
$ = by NICOM; # = by Simpson’s Method
* p< 0.05, compared to baseline
Discussion
While environmental hypothermia is a common medical problem the hemodynamic response to environmental hypothermia has not been studied.(11) Our study allowed us to follow the hemodynamic changes associated with short-term, severe environmental cold exposure, the response to passive rewarming and the cardiovascular physiology of tumo during extreme hypothermia. In both the volunteers and tumo practitioners, exposure to cold was associated with a significant increase in total peripheral resistance (vasoconstriction) with a significant fall in stroke volume and cerebral blood flow. In the volunteers these changes returned to baseline with passive rewarming. It is well known that the first response to environmental hypothermia is an increase in sympathetic tone, leading to intense arteriovenous constriction as a heat-conserving mechanism to decrease blood flow to the skin.(11-13) The 34% increase in TPRI recorded in the volunteer group is in agreement with this concept, and validates the technology used in this study. It is noteworthy that the heart rates remained unchanged while the SVI fell, resulting in a fall in cardiac index. The fall in stroke volume may be consequent to the increased ventricular afterload due to intense vasoconstriction. The fall in cardiac index may be somewhat offset by the increase in basal metabolic rate and oxygen consumption associated with hypothermia.(14) There was a smaller increase in TPRI in the tumo group with cold exposure (21%); this may represent some degree of adaption to hypothermia. Most studies that have investigated the cardiovascular effects of induced hypothermia have been performed following cardiac arrest, where the underlying cardiac disease confounds the hemodynamic picture. (15) However, our findings are similar to those reported by He and colleagues who in an ovine mild-hypothermia model demonstrated that hypothermia was associated with an increase in TPRI and a fall in SVI which was reversed with rewarming. (16) It is noteworthy that in our study cerebral blood flow fell by 26% in the volunteers and 17% in the tumo group. It has previously been reported that cerebral metabolism is depressed 6 to 7% per1°Cdecrease in core temperature; (12,17,18) It is likely that preserved metabolic coupling with cerebrovascular reactivity accounts for the fall in cerebral blood flow.(19) These findings are in contrast to experimental studies which have demonstrated that cooling leads to dilatationof the carotid artery with an increase in cerebral blood flow, while hyperthermia causes cerebral vasoconstriction with a fall in cerebral blood flow.(20)
We were able to identify a single previous study (published in 1985) which investigated the physiologic changes with tumo.(2) Benson and colleagues studies the changes in central and peripheral body temperature in two tumo practitioners. This study was conducted in unheated, uninsulated stone huts in the foothills of the HimalayanMountains. While the rectal temperature of the tumo practitioners remained constant, finger and toe temperature increased by up to 7.2o C. It was postulated that the increase in peripheral skin temperate was due to vasodilation and increased cutaneous blood flow. Remarkably, the air temperature within the hut increased by up to 3.2o C. It is our supposition that the increased hut temperature can only be explained by the exhalation of warm air by the tumo practitioners.
The hemodynamic changes which we noted with tumo were quite remarkable and remain somewhat unexplained. Despite being exposed to sub-zero temperatures the tumo practitioners were able to vasodilate and increase their cardiac output with a marked increase in cerebral blood flow. As the core (and brain) temperature remains unchanged during tumo,(2) and presuming cerebral auto-regulation is intact, this would suggest that the increased cerebral blood flow is a consequences of increased brain activity and metabolic demands. In experimental animal models increased brain activity is associated with intra-brain heat production and increased cerebral blood flow.(21) Furthermore, using single-photon emission computed tomography (SPECT) Newberg et al. demonstrated an increase in cerebral blood flow in Tibetan Buddhists during mediation. (22) In order to explain the physiological changes associated with tumo, basal metabolic rate and oxygen consumption must increase dramatically to account for the increased heat production. We postulate that tumo results in a massive increase in sympathic tone with increased adaptive thermogenesis in brown adipose tissue and skeletal muscle. Mitochondrial uncoupling, the process whereby substrate oxidation is uncoupled from ATP production, results in heat production and is responsible for adaptive thermogenesis.(23-26) Unlike white adipose tissue which acts predominantly as an energy storage depot, brown adipose tissue is a thermogenic organ dissipating energy in the form of heat.(23,26) Brown adipose tissue and skeletal muscle are rich in mitochondria which contain uncoupling protein-1 (UCP-1) which is responsible for adaptive thermogenesis.(23-26) In addition, cold adaption results in an increase in the size of the brown adipose tissue depots.(27) β-adrenergic activation of brown adipose tissue increases the availability of fatty acids for oxidative metabolism and increases UCP-1 activity.(26) We postulate that tumo increases sympathetic activity with increased expression and activation of UCP-1 with uncoupling of fatty acid oxidation in brown adipose tissue and skeletal muscle. It is noteworthy that after performing tumo, practitioners eat large volumes of clarified yak buttermilk ghee alone or with barley flower (tsampa), presumably to restore their brown fat stores. Remarkably ghee is thought to be sacred to Agni (as in Agni-Sara) the Hindu god of fire.
Conclusions:
Exposure to extreme cold resulted in peripheral vasoconstriction and a fall in cardiac output, effects that serve to limit heat loss. Tumo performed in the same environmental conditions resulted in an increase in cardiac output with vasodilatation. Further research of the tumo phenomenon could provide insight into the mind-body connection and its effects on physiologic functions. In addition, tumo induced thermogenesis could potentially have a role in weight loss programs.
ACKNOWLEDGMENTS
We acknowledge the efforts and echocardiographic skills of Alex Levitov, MD without whose help this project would not have been possible. We thank I.V. Arkhipova, the director of the Faraon studio of historical films and the organizer of international research expeditions to theHimalayas, carried out through the project “V poiskakh utrachennykh znanii” (c) (In Search for Lost Knowledge). We acknowledge SonoSite (Fujifilm) Corporation and Cheetah Medical for the equipment support of the study.
CONTRIBUTIONS:
RSM was the principle investigator for this study. RSM designed the study. RSM, ARB, RRB were responsible for the recruitment of subjects and execution of the study. DPB provided technical assistance and performed the echocardiography and carotid Doppler studies. PEM and RSM interpreted and analyzed the data. PEM wrote the first draft of the manuscript. All authors helped to critically revise the draft and approved the final version of the report.
References
1. Yeshe L. The Bliss of Inner Fire: Heart Practice of the Six Yogas of Naropa. Boston: Wisdom Publications; 1998.
2. Benson H, Lehnann JW, Malhotra MS et al. Body temperature changes during the practice of g Tum-mo yoga. Nature 1982; 295:234-236.
3. Lang RM, Bierig M, Devereux RB et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr 2005; 18:1440-1463.
4. Asmer I, Adawi S, Ganaeem M et al. Right ventricular outflow tract systolic excursion: a novel echocardiographic parameter of right ventricular function. European heart journal cardiovascular Imaging 2012; 13:871-877.
5. Tousignant C, Kim H, Papa F et al.Evaluation of TAPSE as a measure of right ventricular ouptut. Can J Anaesth 2012; 59:376-383.
6. Marik PE, Levitov A, Young A et al. The use of NICOM (Bioreactance) and Carotid Doppler to determine volume responsiveness and blood flow redistribution following passive leg raising in hemodynamically unstable patients. Chest 2013; 143:364-370.
7. Keren H, Burkhoff D, Squara P. Evaluation of a noninvasive continuous cardiac output monitoring system based on thoracic bioreactance. Am J Physiol 2007; 293:H583-H589.
8. Raval NY, Squara P, Cleman M et al. Multicenter evaluation of noninvasive cardiac output measurement by bioreactance technique. J Clin Monit Comp 2008; 22:113-119.
9. Squara P, Denjean D, Estagnasie P et al. Noninvasive cardiac output monitoring (NICOM): a clinical validation. Intensive Care Med 2007; 33:1191-1194.
10. Marik PE. Non-invasive cardiac output monitors. A state-of-the-art review. J Cardiothorac Vasc Anesth 2013; 27:121-134.
11. Brown DJ, Brugger H, Boyd J et al. Accidental hypothermia. N Engl J Med 2012; 367:1930-1938.
12. Leikin SM, Korley FK, Wang EE et al. The spectrum of hypothermia: from environmental exposure to therapeutic uses and medical simulation. Disease-A-Month 2012; 58:6-32.
13. Jurkovich GJ. Environmental cold-induced injury. Surg Clin North Am 2007; 87:247-267.
14. van Marken Lichtenbelt WD, Schrauwen P, van De KS et al. Individual variation in body temperature and energy expenditure in response to mild cold. Am J Physiol Endocrinol Metab 2002; 282:E1077-E1083.
15. Kelly FE, Nolan JP. The effects of mild induced hypothermia on the myocardium: a systematic review. Anaesthesia 2010; 65:505-515.
16. He X, Su F, Taccone FS et al. Cardiovascular and microvascular responses to mild hypothermia in an ovine model. Resuscitation 2012; 83:760-766.
17. Rosomoff HL, Holaday DA. Cerebral blood flow and cerebral oxygen consulption during hypothermia. Am J Physiol 1954; 179:85-88.
18. Milde LN. Clinical use of mild hypothermia for brain protection: a dream revisited. J Neurosurg Anesthesiol 1992; 4:211-215.
19. Greeley WJ, Ungerleider RM, Kern FH et al. Effects of cardiopulmonary bypass on cerebral blood flow in neonates, infants, and children. Circulation 1989; 80:I209-I215.
20. Mustafa S, Elgazzar AH, Ismael HN. Influence of hyperthermia on carotid blood flow using 99mTc-HMPAO. Eur J Appl Physiol 2007; 101:257-262.
21. Kiyatkin EA. Brain temperaturefluctuations during physiological and pathological conditions. Eur J Appl Physiol 2007; 101:3-17.
22. Newberg A, Alavi A, Baime M et al. The measurement of regional cerebral blood flow during the complex cognitive task of meditation: a preliminary SPECT study. Psychiatry Research 2001; 106:113-122.
23. van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM et al. Cold-activated brown adipose tissue in healthy men. N Engl J Med 2009; 360:1500-1508.
24. Wijers SL, Schrauwen P, Saris WH et al. Human skeletal muscle mitochondrial uncoupling is associated with cold induced adaptive thermogenesis. PloS ONE 2008; 3:e1777.
25. van den Berg SA, van Marken LW, Willems van DK et al. Skeletal muscle mitochondrial uncoupling, adaptive thermogenesis and energy expenditure. [Review]. Current Opinion in Clinical Nutrition & Metabolic Care 2011; 14:243-249.
26. Richard D, Picard F. Brown fat biology and thermogenesis. Frontiers in Bioscience 2011; 16:1233-1260.
27. Bukowiecki L, Collet AJ, Follea N et al. Brown adipose tissue hyperplasia: a fundamental mechanism of adaptation to cold and hyperphagia. Am J Physiol 1982; 242:E353-E359.
Файлы для скачивания: